The DS CGMP rule needs you to visually take a look at the provider's invoice, warranty, or certification, and each quick container or grouping of immediate containers, in the cargo of parts.
Once that level is decided, companies could establish appropriate corresponding analytical examination requirements. Firms may possibly then use the analytical tests for preservative information at batch release and through the shelf life of heaps on security. References:
All personnel involved in the manufacturing process need to be appropriately properly trained and experienced to carry out their Employment.
All staff are expected to strictly adhere to manufacturing procedures and laws. A current GMP instruction must be carried out by all staff to completely understand their roles and responsibilities. Assessing their functionality assists Enhance their productiveness, effectiveness, and competency.
The 5Ps of GMP will be the critical features to think about when applying successful guidelines for your online business. These 5Ps compose of the next:
Case in point two. A manufacturer who hires a contractor to accomplish unique operations in the scope of the company's responsibilities under the DS CGMP rule is answerable for complying Using the requirements linked to the contracted operation.
Law corporations seeking pharmaceutical consulting business abilities in the remediation of warning letters, consent decrees, 483’s or import bans
Premises should advertise cleanliness all of the time in order to avoid cross-contamination, mishaps, or simply fatalities. All products really should be put or saved appropriately and calibrated routinely to make certain They can be suit for the objective of manufacturing regular benefits to avoid the danger of equipment failure.
What exactly are examples of h2o that will turn into a component of the dietary nutritional supplement? Samples of h2o which could turn into a ingredient of the dietary nutritional supplement consist of water that contacts factors, dietary supplements, or any Make contact with floor.
Any Uncooked resources Employed in the manufacture of drugs has to be of verifiable high quality and have to satisfy all relevant regulatory requirements. This incorporates Energetic pharmaceutical ingredients (APIs) and any excipients.
“A GMP is often a procedure for guaranteeing that products and solutions are continuously made and controlled Based on quality standards. It is actually built to reduce the risks associated with any pharmaceutical generation that cannot be removed by tests the ultimate product or service”. Good manufacturing practice (gmp)
What does the DS CGMP rule involve me to complete with turned down factors, packaging, and labels, and with rejected products and solutions gained for packaging or labeling as a dietary complement? The DS CGMP rule calls for you to clearly identify, hold, and Handle below a quarantine technique for suitable disposition any ingredient, packaging, and label, and any solution you receive for packaging or labeling as a dietary complement, that is definitely turned down and unsuitable to be used in manufacturing, packaging, read more or labeling functions.
The standard of made products is extremely regulated as it may possibly pose destructive well being pitfalls to individuals as well as the setting. Weak hygiene, temperature-control, cross-contamination, and adulteration in almost any step in the manufacturing approach are some examples of how a produced products that doesn’t follow GMP restrictions read more can convey lethal consequences to people. See GMP regulation and preamble resources by nation here.
Validated analytical techniques are desired for tests every batch, which includes validation batches. The Agency would also count on the producer to make use of a validation protocol that includes a review and remaining report soon after multiple batches are finished, Although the earlier batches might happen to be dispersed or used in the completed drug products.
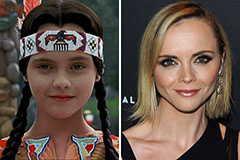 Christina Ricci Then & Now!
Christina Ricci Then & Now!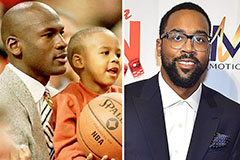 Marcus Jordan Then & Now!
Marcus Jordan Then & Now! Raquel Welch Then & Now!
Raquel Welch Then & Now! Bill Cosby Then & Now!
Bill Cosby Then & Now! Kerri Strug Then & Now!
Kerri Strug Then & Now!